Corrosion Behavior and Preventive Measures of Metal Equipment During Cleaning Operations
Category: Corporate News
Published Time: 2024-05-17
Summary: Over the past century, the food industry, which has been led and derived from the dairy and beverage sectors, has gradually become more complete in terms of its industrial chain. Currently, China's food industry has entered a phase of extremely rapid development, and a series of associated consumables such as cleaning agents and disinfectants are also moving towards improved performance.
01 Introduction

Over the past century, the food industry, which has been led and derived from the dairy and beverage sectors, has gradually become more complete in terms of its industrial chain. Currently, China's food industry has entered a phase of extremely rapid development, and a series of associated consumables such as cleaning agents and disinfectants are also moving towards improved performance.
Cost-effective, eco-friendly, residue-free, and non-corrosive sanitizing products now command significant market share. This article will analyze prevalent corrosion mechanisms affecting metal equipment in food processing from a chemical perspective.
02 The principle of metal chemical corrosion

In general, there are three main pathways for metal components to be corroded by aqueous solutions: hydrogen evolution corrosion, oxygen absorption corrosion, and electrochemical corrosion.
1. Hydrogen Evolution Corrosion
Hydrogen evolution corrosion usually occurs in substrates with ordinary anti-corrosion ability in acidic environments. For example, when active non-stainless steel substrates such as aluminum, iron, steel, and carbon steel come into direct contact with acid solutions like sulfuric acid and nitric acid, the metals in them react with hydrogen ions in the acid, causing the substrates to be corroded and hydrogen gas to be released at the same time.
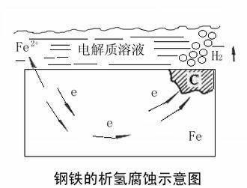
Note: Hydrogen evolution corrosion rarely occurs in isolation during routine production and daily operations; it only occurs in large quantities under circumstances such as incorrect use of materials and tools.
2. Oxygen Absorption Corrosion
Oxygen absorption corrosion mostly occurs in neutral or alkaline humid environments. When substrates with ordinary anti-corrosion ability come into contact with both water and air, such corrosion is highly likely to occur. The rusting of various iron products is a typical case of oxygen absorption corrosion.
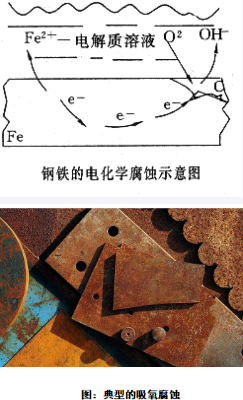
3. Electrochemical Corrosion
Electrochemical corrosion is one of the most common types of chemical corrosion in current industrial production and daily life. Unlike ordinary metal corrosion, it occurs when two different metals are simultaneously immersed in a corrosive solution. In this case, one metal acts as the anode and the other as the cathode, and a potential difference is generated between them. The anode, also known as the sacrificial metal, corrodes and deteriorates faster than when it corrodes alone, while the cathode corrodes and deteriorates more slowly than in other corrosion modes.
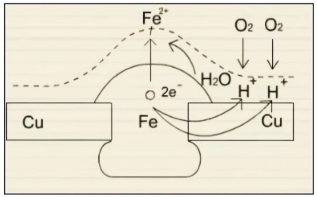
Three essential requirements for electrochemical corrosion:
①Electrically dissimilar metals must contain components capable of electrochemical reactions with an electrolyte;
②Electrical contact must exist between the metals;
③Immersion in a common electrolyte must occur.
The current market exhibits a diverse spectrum of performance requirements for metallic materials, predominantly iron-based alloys, which frequently satisfy both condition ① and ②. Since acidic solutions in cleaning solution containment systems inevitably come into contact with material components, the risk of electrochemical corrosion increases significantly when improper materials are selected.

Figure: Electrochemical corrosion of dissimilar metal contact
In addition to the three basic types of corrosion mentioned above, complex corrosion scenarios are often caused by the combined action of multiple corrosive conditions or various factors (such as chloride ions and temperature).A representative case is chloride-induced pitting corrosion. Chloride ions demonstrate particularly severe corrosive effects on both 304 and 316L stainless steels, where temperature acts as a secondary contributing factor.

Figure: Corrosion at the interface of a high-temperature acid storage equipment
In the cleaning water samples collected by our team from multiple pastoral sites, the chloride ion concentration was comparable to that of local groundwater, and far above the minimum chloride ion level at which stainless steel corrosion occurs. Among them, a small number of storage tanks also had inconsistent materials used in different components such as the tank body, joints, temperature probes, and welding materials. This is more likely to intensify the occurrence of electrochemical corrosion in acidic environments, and may even lead to leakage, loosening, and seepage at these connections, thereby posing certain safety hazards.

Figure: Severe pitting corrosion of 304 stainless steel
03 Preventive Measures

Once corrosion occurs, the consequences are difficult to reverse. In the routine cleaning processes of dairy and beverage industry equipment, the use of various water-based cleaning agents is inevitable. Therefore, in terms of the conditions for equipment corrosion, the most basic contact condition is already unavoidable. Thus, it is particularly important to slow down and prevent the occurrence of corrosion through other means.
1. Avoid using cleaning water with high chloride ion content for equipment cleaning and soaking as much as possible. For 304 stainless steel applications, the chloride ion concentration should be maintained within 0-200 ppm. If conditions permit, water with chloride ion content ≤25ppm can be used for long - term cleaning purposes.
2. Avoid using metal components with significant material differences in equipment that come into contact with each other to prevent the formation of circuits that can cause electrochemical corrosion. If material differences cannot be avoided, a corrosion-resistant non-metallic material should be interposed between the different metal materials.
3. Control the usage temperature and avoid long-term contact between strong acid solutions and equipment at high temperatures as much as possible.
4. Select specialty cleaners with corrosion inhibiting properties for equipment protection.
Keywords: Corrosion Behavior and Preventive Measures of Metal Equipment During Cleaning Operations
Related News
Corporate News
-
Klean Empowerment Bootcamp | Three Days of Intensive Training: Unlocking Advanced Strategies in Management and Sales
Time:2025-06-07
-
University-Enterprise Synergy Cultivates Elite Talents | Klean and Northwestern Polytechnical University jointly established employment and internship base
Time:2025-04-27
-
The cornerstone of food safety – Cleanliness is the lifeline of the food industry
Time:2024-08-01
-
Corrosion Behavior and Preventive Measures of Metal Equipment During Cleaning Operations
Time:2024-05-17
-
CIP Cleaning in the Food Industry: The “Secrets” You Never Knew!
Time:2024-04-10
-
How to properly use disinfectants
Time:2025-05-12
-
How to properly store alkaline and acidic cleaning agents?
Time:2025-05-12
-
What are the usage methods and precautions for alkaline cleaning agents?
Time:2025-05-12
-
How to properly use acidic cleaning agents to avoid danger?
Time:2025-05-12
-
Application scenarios of acidic cleaning agents
Time:2025-05-12
Industry Trends
-
The State Administration for Market Regulation (SAMR) has introduced 37 key measures to support the development of private enterprises.
Time:2025-04-17
-
Green consumption new fashion: Master Kong's low-carbon products debut at Shanghai Carbon Expo
Time:2025-06-17
-
From Guizhou to the world - Ciningji C+ makes its debut at the Guizhou prickly pear industry event
Time:2025-06-17
-
Four departments jointly issue agricultural disaster prevention and mitigation plan for flood season, striving for a bumper harvest of grain and agriculture
Time:2025-05-13
-
Warmly welcome! The Dairy Industry Conference, D20 Forum, and Exhibition will be held in Xiamen from July 13-15.
Time:2025-05-27
-
What is the difference between alkaline and acidic cleaning agents?
Time:2025-05-12
-
What are the applications of alkaline cleaning agents?
Time:2025-05-12
-
Which metal materials cannot be cleaned with acidic cleaning agents?
Time:2025-05-12
-
How should I handle an acid cleaning agent spill on my skin?
Time:2025-05-12
-
How corrosive are acidic cleaners?
Time:2025-05-12








 陕公网安备61042202000152号
陕公网安备61042202000152号